All medical devices prior to placing on the Moldavian market are subject to mandatory marketing authorization, which is performed:
- when the medical device has the CE marking: by notification,
- when the medical device has not CE marking: by national conformity assessment and registration.
In both cases foreign manufacturer must designate an Authorized representative at the territory of Moldova. Authorized representative must be a resident of Moldova, should have the relevant written authorization from manufacturer (Agreement and/or Power of Attorney), has the right to initiate specific regulatory procedures and acts as a contact point between competent authorities and manufacturer.
Medical devices authorized to be placed on the EU market (having CE marking) undergo the simplified procedure for admission to the market by notification. The legislation determines that since such devices have already passed the conformity assessment, there is no need to repeat these procedures.
The device is placed on the market without the national SM conformity marking, however, before placing the product on the market it is necessary to perform a number of administrative procedures.
Medical devices that were not previously placed on the EU market must undergo the conformity assessment procedure in accordance with the legislation of Moldova, and then they are entered in the State register of medical devices. It is necessary:
- To designate the Authorized representative in Moldova, for which purpose the manufacturer must transfer the necessary rights to the resident legal entity of Moldova by means of the Power of Attorney or Contract;
- To prepare national labeling and instructions for use (user manual);
- To fill in and submit the Technical documentation, formed depending on the class of medical device;
- To apply for registration of the medical device by notifying the Agency for medicinal products and medical devices;
- Place the national symbol (mark) of conformity “SM”.
Authorized representative of the manufacturer
If the manufacturer of the medical device is not a resident of the Republic of Moldova, then such manufacturer must designate the Authorized representative. The Authorized representative is designated by the Contract or Power of Attorney. The Authorized representative may be entitled to initiate conformity assessment procedures.
Responsibilities of the Authorized representative are:
- to place his name and address on the medical devices, which have passed the marketing authorization procedure;
- to register with the Agency for medicinal products and medical devices and to provide the description of the products that are the subject of his activities for entering data into Agency’s database;
- to receive information about incidents from the Agency and to take the necessary actions;
- to take the necessary actions in case of detection of medical devices without proper labeling;
- to keep the documentation and provide the Agency with access to it during 5 years from the time of placing the medical device on the market:
- declaration of conformity;
- technical documentation;
- changes in technical documentation;
- decisions and documents of the conformity assessment body.
Package labeling and instructions for use (user manual)
The information on the product labeling may be indicated in the form of international symbols. The labeling of the medical device, the instructions for use (user manual) should be provided to users and patients in Romanian. Thus, on the labeling of the medical device that contains international symbols, it is necessary to indicate in Romanian:
- the name or brand name of the manufacturer, name and address of the authorized representative in the Republic of Moldova;
- the name of the medical device and other data necessary to identify the device and the contents of the package;
- if the device is made to order, the following should be indicated on it: “dispozitiv fabricat la comandă”;
- if the device is intended for clinical studies, the following should be indicated on it: “exclusiv pentru investigaţii clinice”;
- any special conditions of storage and/or handling;
- any special operational instructions;
- any warnings and/or precautions to be taken;
- information that the product contains substances derived from human blood, if applicable.
The national conformity mark “SM” (Securitatea conform cerinţelor esenţiale Moldova) indicates that the manufacturer or his Authorized representative is responsible for placing this mark, has checked the conformity of the products to all main requirements applied in the technical regulations and that the products has been subjected to conformity assessment procedures provided by all applicable technical regulations.
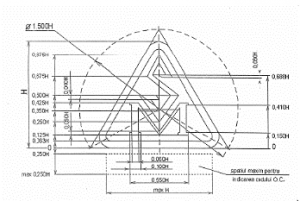
| The graphic representation of the SM mark | The specifications (proportions) applied to the SM mark |
 |
 |
|
The graphic representation of the SM mark |
The specifications (proportions) applied to the SM mark |
The national conformity mark should be followed by the identification number of the conformity assessment body, if the specified authority has participated in the conformity assessment procedure. The identification number of the authority shall be placed by the authority itself, or, at the direction of that authority, by the manufacturer or his Authorized representative.
Cratia provides professional services of registration of medical devices in Moldova. We have an excellent knowledge of national legislation, necessary experience and resources.
To start cooperation or get advice, please contact us by phone: +38 068 064-78-31, +38 044 223-61-67, by e-mail: info@cratia.ua, or visit our office.
